Mg no3 2 k3po4 mg3 po4 2 kno3 – Prepare to delve into the captivating realm of chemistry as we unravel the intriguing interactions between Mg(NO3)2, K3PO4, Mg3(PO4)2, and KNO3. This quartet of compounds holds a wealth of secrets, and we’re eager to unlock their mysteries.
As we embark on this scientific adventure, we’ll explore the chemical reactions that govern these substances, unravel their solubility and precipitation behaviors, and delve into the fascinating world of solution chemistry. Along the way, we’ll uncover their practical applications, revealing how these compounds play vital roles in various industries.
Chemical Reactions
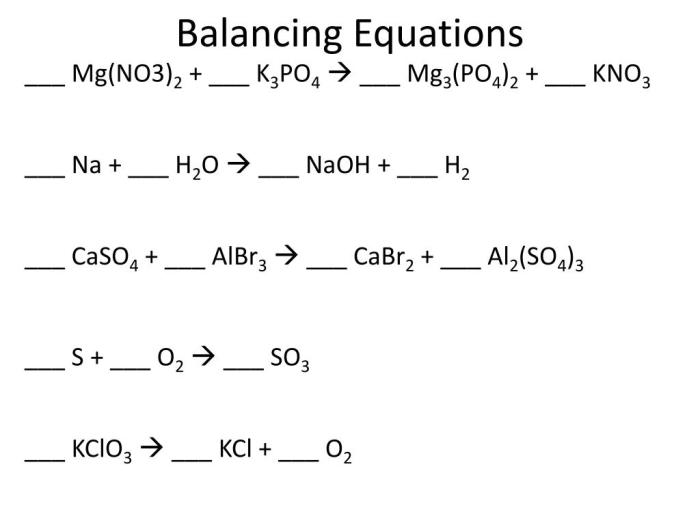
In a chemical reaction, atoms and molecules undergo changes to form new substances. The reaction between Mg(NO3)2, K3PO4, Mg3(PO4)2, and KNO3 is a double displacement reaction where the cations and anions of the reactants switch places to form new products.
Chemical Equation
The balanced chemical equation for this reaction is:
Mg(NO3)2 + 2K3PO4 → Mg3(PO4)2 + 2KNO3
In this equation, the reactants are Mg(NO3)2 and K3PO4, while the products are Mg3(PO4)2 and KNO3.
Stoichiometry
The stoichiometry of the reaction indicates the mole ratios of the reactants and products. From the balanced equation, we can see that:
- 1 mole of Mg(NO3)2 reacts with 2 moles of K3PO4.
- 1 mole of Mg(NO3)2 produces 1 mole of Mg3(PO4)2.
- 2 moles of K3PO4 produces 2 moles of KNO3.
This information can be used to calculate the amounts of reactants and products involved in the reaction.
Solubility and Precipitation
Solubility refers to the ability of a substance to dissolve in a solvent, forming a homogeneous mixture called a solution. The extent of solubility is typically expressed in terms of concentration, which indicates the amount of solute (the substance being dissolved) present in a given amount of solvent or solution.
Precipitation is the process by which a solid forms from a solution. This occurs when the concentration of the solute exceeds the solubility limit, causing the excess solute to crystallize out of the solution.
When studying the chemical reactions of mg no3 2 k3po4 mg3 po4 2 kno3, it’s important to have a strong foundation in basic vocabulary. A helpful resource for expanding your word knowledge is the list of fry’s first 100 words . This list provides a comprehensive overview of essential words for effective communication.
By incorporating these words into your study of mg no3 2 k3po4 mg3 po4 2 kno3, you’ll enhance your understanding and ability to express complex chemical concepts.
Solubility of Compounds
The solubility of Mg(NO3)2, K3PO4, Mg3(PO4)2, and KNO3 in water varies depending on the temperature and other factors. At room temperature, the approximate solubility of these compounds in water is as follows:
- Mg(NO3)2: Highly soluble
- K3PO4: Moderately soluble
- Mg3(PO4)2: Slightly soluble
- KNO3: Highly soluble
Precipitation Reactions, Mg no3 2 k3po4 mg3 po4 2 kno3
When these compounds are mixed in certain combinations, precipitation reactions can occur. For example, if a solution of Mg(NO3)2 is mixed with a solution of K3PO4, a precipitate of Mg3(PO4)2 will form because the solubility limit of Mg3(PO4)2 is exceeded.
Mg(NO3)2(aq) + K3PO4(aq) → Mg3(PO4)2(s) + KNO3(aq)
Similarly, if a solution of Mg3(PO4)2 is mixed with a solution of KNO3, no precipitate will form because both compounds are highly soluble in water.
Solution Chemistry
Solution chemistry deals with the behavior of chemical species in solution. In this context, we have a solution containing Mg(NO3)2, K3PO4, Mg3(PO4)2, and KNO3. Understanding the solution’s properties is crucial for various chemical processes.
Molarity of the Solution
Molarity is a measure of the concentration of a solution, expressed as the number of moles of solute per liter of solution. To calculate the molarity of the given solution, we need to know the number of moles of each solute and the total volume of the solution.
- Number of moles of Mg(NO3)2: [Mg(NO3)2] (in moles)
- Number of moles of K3PO4: [K3PO4] (in moles)
- Number of moles of Mg3(PO4)2: [Mg3(PO4)2] (in moles)
- Number of moles of KNO3: [KNO3] (in moles)
Total volume of the solution: V (in liters)
Molarity of the solution: [Total moles of solute] / V
Ionic Strength
Ionic strength is a measure of the concentration of ions in a solution. It is calculated using the following formula:
Ionic strength (I) = 1/2 – Σ(Ci – Zi^2)
- Ci: Concentration of ion i (in moles per liter)
- Zi: Charge of ion i
For the given solution, we need to consider the following ions:
- Mg2+: Concentration [Mg2+]
- NO3-: Concentration [NO3-]
- K+: Concentration [K+]
- PO43-: Concentration [PO43-]
Once we have the concentrations of each ion, we can calculate the ionic strength using the formula above.
Colligative Properties
Colligative properties are properties of solutions that depend on the concentration of the solute particles, regardless of their chemical nature. These properties include boiling point elevation and freezing point depression.
Boiling Point Elevation
Boiling point elevation is the increase in the boiling point of a solution compared to the boiling point of the pure solvent. The boiling point elevation (ΔTb) is calculated using the following formula:
ΔTb = Kb – m
- Kb: Boiling point elevation constant of the solvent (in K kg/mol)
- m: Molality of the solution (in moles of solute per kilogram of solvent)
For the given solution, we need to know the molality of the solution and the boiling point elevation constant of the solvent.
Freezing Point Depression
Freezing point depression is the decrease in the freezing point of a solution compared to the freezing point of the pure solvent. The freezing point depression (ΔTf) is calculated using the following formula:
ΔTf = Kf – m
- Kf: Freezing point depression constant of the solvent (in K kg/mol)
- m: Molality of the solution (in moles of solute per kilogram of solvent)
For the given solution, we need to know the molality of the solution and the freezing point depression constant of the solvent.
Applications
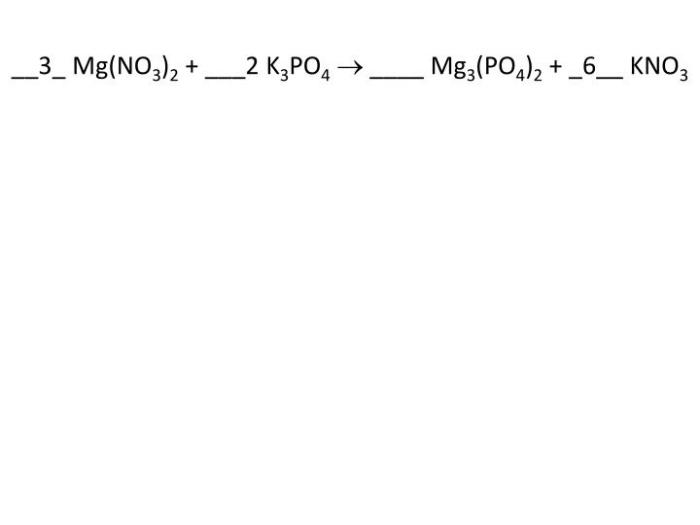
The compounds Mg(NO3)2, K3PO4, Mg3(PO4)2, and KNO3 possess unique properties that render them valuable in various fields. These compounds find applications in agriculture, manufacturing, and scientific research.
Agriculture
- Mg(NO3)2:As a source of magnesium and nitrogen, Mg(NO3)2 enhances crop growth and yield. It improves soil fertility and promotes chlorophyll production, essential for photosynthesis.
- K3PO4:A potassium and phosphorus fertilizer, K3PO4 aids in root development, flowering, and fruit production. It enhances crop quality and resistance to pests and diseases.
Manufacturing
- Mg3(PO4)2:Used in the production of fire-resistant materials, Mg3(PO4)2 exhibits excellent thermal insulation properties. It finds applications in construction, aerospace, and automotive industries.
- KNO3:Employed as a fertilizer and in the production of explosives, KNO3 is also used in the manufacturing of glass, ceramics, and dyes.
Scientific Research
- Mg(NO3)2:Serves as a precursor for the synthesis of various magnesium-based compounds used in biomedical research and materials science.
- K3PO4:Used in biochemistry and molecular biology as a buffer solution to maintain a stable pH in experiments.
Essential Questionnaire: Mg No3 2 K3po4 Mg3 Po4 2 Kno3
What is the chemical equation for the reaction between Mg(NO3)2 and K3PO4?
Mg(NO3)2 + 2K3PO4 → Mg3(PO4)2 + 2KNO3
What is the solubility of Mg3(PO4)2 in water?
Mg3(PO4)2 is insoluble in water.
What is the molarity of a solution containing 0.1 moles of Mg(NO3)2 in 1 liter of water?
0.1 M