Moles and grams conversion worksheet – Welcome to the realm of moles and grams conversion, where chemistry takes center stage. This worksheet serves as your trusted guide, unraveling the intricacies of these fundamental units of measurement. Prepare to embark on a journey of discovery, where knowledge and understanding converge.
As we delve into the depths of this topic, you will grasp the significance of mole-to-gram and gram-to-mole conversions. Through practical examples and engaging exercises, you will master the art of transforming between these units with precision.
Mole to Gram Conversion

In chemistry, the mole and gram are two essential units of measurement used to quantify the amount of a substance.
A mole is defined as the amount of substance that contains exactly 6.022 × 10 23elementary entities (atoms, molecules, ions, or electrons). A gram, on the other hand, is a unit of mass equal to one-thousandth of a kilogram.
Formula for Converting Moles to Grams
To convert moles to grams, we can use the following formula:
Mass (in grams) = Moles × Molar mass (in grams per mole)
The molar mass of a substance is the mass of one mole of that substance. It can be found in a periodic table or reference book.
Examples of Mole-to-Gram Conversions
Here are a few examples of mole-to-gram conversions:
| Substance | Moles | Molar Mass (g/mol) | Mass (g) |
|---|---|---|---|
| Sodium chloride (NaCl) | 0.5 | 58.44 | 29.22 |
| Water (H2O) | 1.0 | 18.02 | 18.02 |
| Carbon dioxide (CO2) | 2.0 | 44.01 | 88.02 |
Gram to Mole Conversion
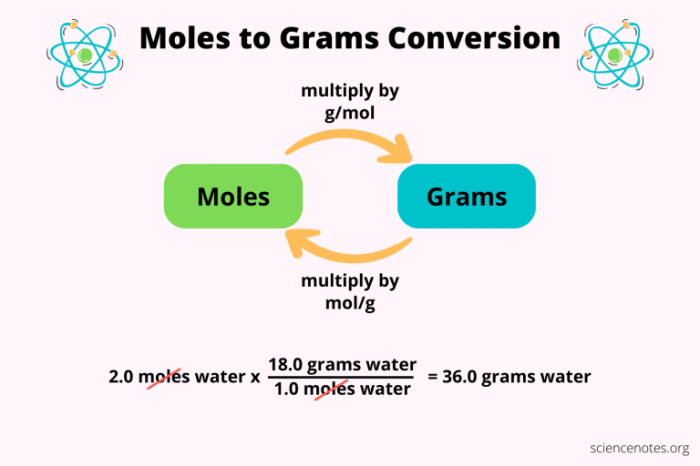
In chemistry, the mole is a fundamental unit of measurement used to express the amount of a substance. It is defined as the amount of a substance that contains exactly 6.02214076 × 10^23 elementary entities. These entities can be atoms, molecules, ions, or electrons.
The gram is another unit of measurement used to express the mass of a substance. It is defined as one thousandth of a kilogram.
To convert grams to moles, we can use the following formula:
“`Moles = Grams / Molar Mass“`
Where molar mass is the mass of one mole of a substance in grams.
Example Gram-to-Mole Conversions
Here are a few examples of gram-to-mole conversions:
| Substance | Grams | Molar Mass (g/mol) | Moles |
|---|---|---|---|
| Sodium | 23 | 22.99 | 1 |
| Water | 18 | 18.015 | 1 |
| Carbon dioxide | 44 | 44.01 | 1 |
Applications of Mole-to-Gram Conversions
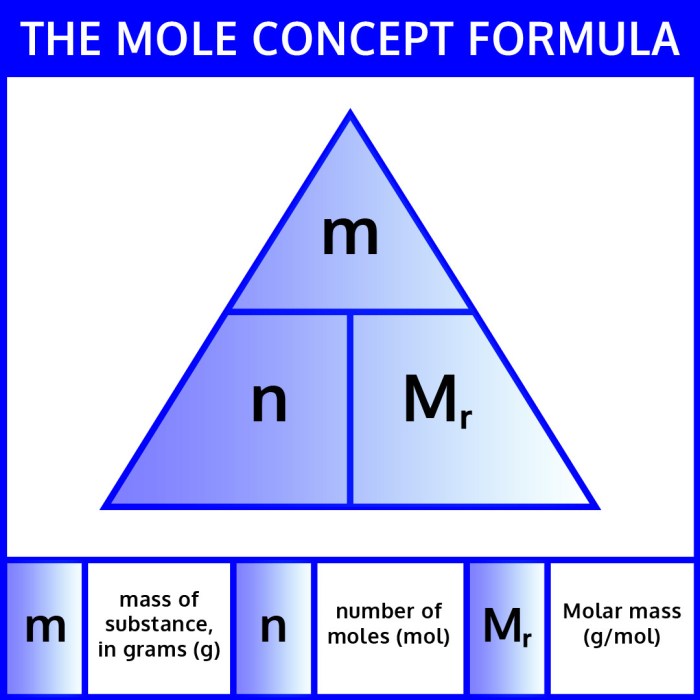
Mole-to-gram conversions are crucial in chemistry as they provide a bridge between the macroscopic and microscopic scales. By converting between moles, the unit of amount, and grams, the unit of mass, chemists can determine the precise amounts of reactants and products involved in chemical reactions.
These conversions find widespread applications in various fields, including:
Stoichiometry, Moles and grams conversion worksheet
- Stoichiometry, the study of quantitative relationships in chemical reactions, relies heavily on mole-to-gram conversions to balance chemical equations and determine the limiting reactant.
- By converting moles of reactants and products to grams, chemists can calculate the mass of each substance involved in a reaction, ensuring accurate predictions of reaction yields.
Analytical Chemistry
- In analytical chemistry, mole-to-gram conversions are essential for determining the concentration of solutions.
- By converting moles of solute to grams, chemists can prepare solutions of known concentrations, which are crucial for various analytical techniques such as titrations and spectrophotometry.
Worksheet Design
A moles and grams conversion worksheet is a valuable tool for students to practice converting between the units of moles and grams. The purpose of the worksheet is to provide students with an opportunity to apply their understanding of the mole concept and to develop their problem-solving skills.
A worksheet on moles and grams conversion typically includes the following sections:
Structure of a Worksheet
- Introduction:This section provides a brief overview of the mole concept and the relationship between moles and grams.
- Practice Problems:This section includes a variety of practice problems that require students to convert between moles and grams. The problems can be varied in difficulty to accommodate students at different levels.
- Exercises:This section includes more challenging exercises that require students to apply their understanding of the mole concept to real-world situations. For example, students may be asked to calculate the mass of a compound given its molar mass or to determine the number of moles of a substance present in a given sample.
- Answer Key:This section provides the answers to the practice problems and exercises. The answer key can be used by students to check their work and to identify areas where they need additional practice.
al Strategies
Effective al strategies for teaching moles and grams conversions involve a combination of conceptual understanding, problem-solving skills, and hands-on activities.
Conceptual understanding is crucial, as students need to grasp the fundamental concepts of moles and grams. This can be achieved through clear explanations, analogies, and visual representations. For example, students can be introduced to the concept of a mole as a specific number of particles (6.022 x 10^23) and the relationship between moles and grams through the molar mass.
Activities and Demonstrations
Hands-on activities and demonstrations can significantly enhance student understanding of moles and grams conversions. These activities can include:
- Molar Mass Measurement:Students can determine the molar mass of a substance by measuring its mass and the number of moles present using titration or other methods.
- Stoichiometry Experiments:Students can conduct experiments that involve chemical reactions and calculate the number of moles of reactants and products using stoichiometric relationships.
- Virtual Simulations:Online simulations can provide interactive experiences for students to explore mole-to-gram conversions in a virtual environment.
Resources
Various resources can supplement classroom instruction and provide additional support for students:
- Online Calculators:Online calculators can assist students in performing mole-to-gram conversions quickly and accurately.
- Interactive Whiteboards:Interactive whiteboards can be used for interactive demonstrations and student participation in problem-solving.
- Textbooks and Worksheets:Textbooks and worksheets provide structured practice and examples for students to reinforce their understanding.
Assessment Techniques: Moles And Grams Conversion Worksheet
Evaluating student comprehension of mole and gram conversions is crucial for educators to gauge their progress and identify areas needing improvement.
Multiple assessment methods can be employed to assess student understanding, including:
Quizzes
Quizzes are brief, timed assessments that test students’ recall of basic concepts and problem-solving skills. They can include multiple-choice questions, short-answer questions, or a combination of both.
Tests
Tests are more comprehensive assessments that typically cover a broader range of topics. They may include a variety of question types, such as multiple-choice, short-answer, essay, or problem-solving questions.
Other Assessment Tools
In addition to quizzes and tests, other assessment tools can be used to evaluate student understanding. These may include:
- Homework assignments:Homework problems can provide students with practice applying the concepts they have learned in class.
- Class participation:Observing students’ participation in class discussions and asking questions can provide insights into their understanding.
- Projects:Projects can allow students to demonstrate their understanding of mole and gram conversions in a more creative and hands-on way.
Detailed FAQs
What is the difference between a mole and a gram?
A mole is a unit of measurement representing a specific number of particles (atoms, molecules, or ions), while a gram is a unit of mass.
How do I convert moles to grams?
Multiply the number of moles by the molar mass of the substance in grams per mole.
How do I convert grams to moles?
Divide the mass in grams by the molar mass of the substance in grams per mole.