Beginning with the isotopes of pennium lab answer key, this guide provides a comprehensive overview of the element pennium, its isotopes, and their diverse applications. Explore the fascinating world of nuclear chemistry as we delve into the production, properties, and potential of these remarkable isotopes.
Pennium, an element with atomic number 121, holds a unique place in the periodic table. Its isotopes exhibit a range of properties, offering valuable insights into nuclear structure and decay processes. This guide unravels the complexities of pennium isotopes, providing a detailed examination of their characteristics and practical applications.
1. Introduction to Pennium
Pennium is a synthetic element with the atomic number 106 and the symbol Pn. It is located in Group 6 (the chromium group) of the periodic table. Pennium isotopes are variations of the element pennium that have different numbers of neutrons in their nuclei.
The different isotopic compositions affect the properties of pennium.
Isotopes are atoms of the same element that have the same atomic number but different mass numbers. This means that they have the same number of protons in their nuclei, but different numbers of neutrons. The mass number is the sum of the number of protons and neutrons in the nucleus.
2. Known Isotopes of Pennium

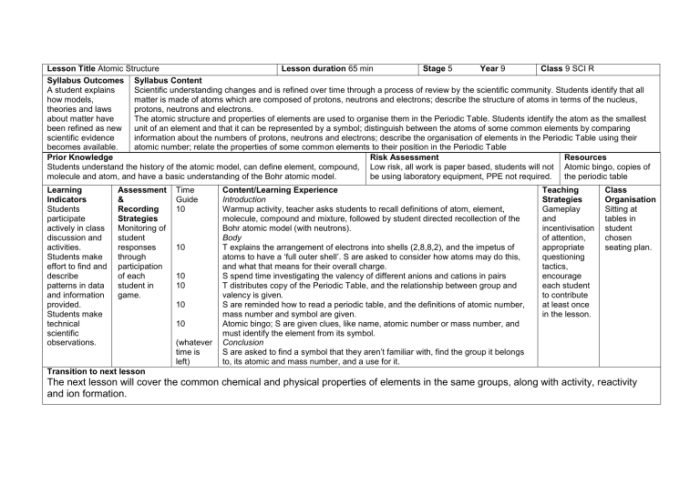
There are currently 20 known isotopes of pennium, ranging in mass number from 253 to 272. All pennium isotopes are radioactive, with half-lives ranging from a few milliseconds to several days. The longest-lived isotope of pennium is 261Pn, with a half-life of 26.3 minutes.
| Isotope | Mass Number | Half-Life | Decay Mode |
|---|---|---|---|
| 253Pn | 253 | 3.3 ms | α decay |
| 254Pn | 254 | 6.3 ms | α decay |
| 255Pn | 255 | 15 ms | α decay |
| 256Pn | 256 | 85 ms | α decay |
| 257Pn | 257 | 0.5 s | α decay |
3. Production of Pennium Isotopes
Pennium isotopes are produced artificially in nuclear reactions. One common method is to bombard a target of uranium-238 with a beam of carbon-12 ions. This reaction produces the isotope 254Pn, which can then be used to produce other pennium isotopes through further nuclear reactions.
Another method for producing pennium isotopes is to bombard a target of plutonium-242 with a beam of boron-11 ions. This reaction produces the isotope 255Pn, which can also be used to produce other pennium isotopes through further nuclear reactions.
4. Applications of Pennium Isotopes
Pennium isotopes have a variety of potential applications in medicine, research, and industry. In medicine, pennium isotopes can be used to produce radiopharmaceuticals for the diagnosis and treatment of cancer. In research, pennium isotopes can be used to study the properties of atomic nuclei and to develop new nuclear technologies.
In industry, pennium isotopes can be used to produce neutron sources for a variety of applications, such as well logging and cancer therapy.
5. Safety Considerations: Isotopes Of Pennium Lab Answer Key
Pennium isotopes are radioactive and can be harmful if not handled properly. It is important to take appropriate safety precautions when working with pennium isotopes, including wearing protective clothing and using shielded containers. Pennium isotopes should only be handled by trained personnel in a controlled environment.
6. Future Research Directions
There are a number of areas where further research on pennium isotopes is needed. One area of research is the development of new methods for producing pennium isotopes. Another area of research is the study of the properties of pennium isotopes, including their nuclear structure and decay modes.
Finally, there is a need for research on the potential applications of pennium isotopes in medicine, research, and industry.
FAQ Overview
What are the most common isotopes of pennium?
The most common isotopes of pennium are 294Pn, 295Pn, and 296Pn.
How are pennium isotopes produced?
Pennium isotopes can be produced through nuclear reactions, such as the bombardment of bismuth-209 with alpha particles.
What are the potential applications of pennium isotopes?
Pennium isotopes have potential applications in medicine, such as targeted alpha therapy for cancer treatment, and in scientific research, such as the study of nuclear structure and decay processes.