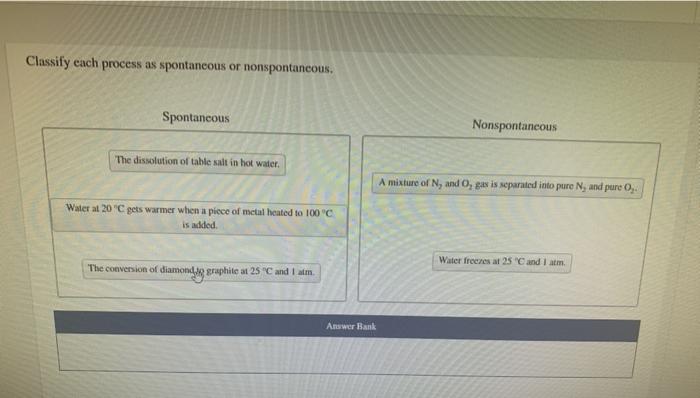
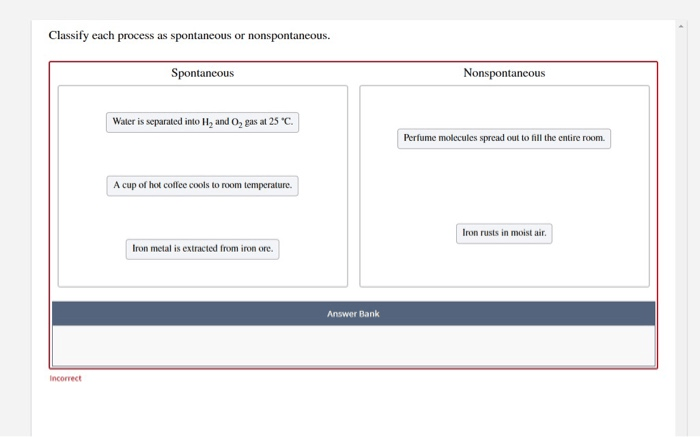
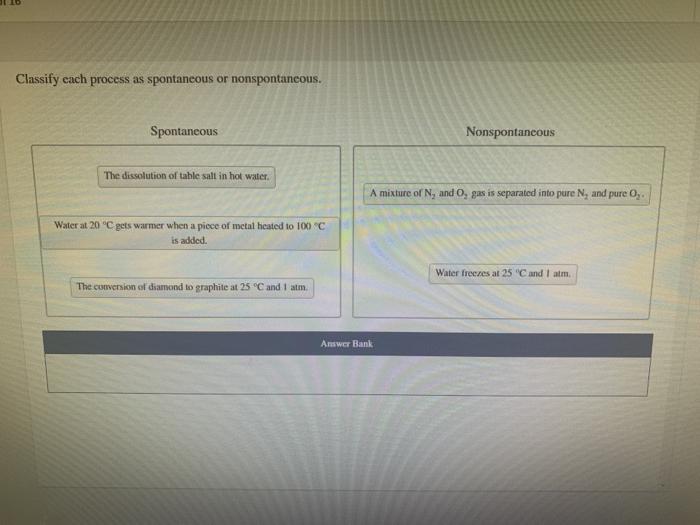
Classify each process as spontaneous or nonspontaneous. This fundamental concept in chemistry unveils the driving forces behind chemical reactions, revealing the intricate interplay between energy, entropy, and spontaneity. Delving into the realm of thermodynamics and reaction kinetics, we embark on a journey to unravel the factors that govern the spontaneity of chemical transformations, shedding light on their practical applications in diverse fields.
Process Classification: Spontaneous vs. Nonspontaneous
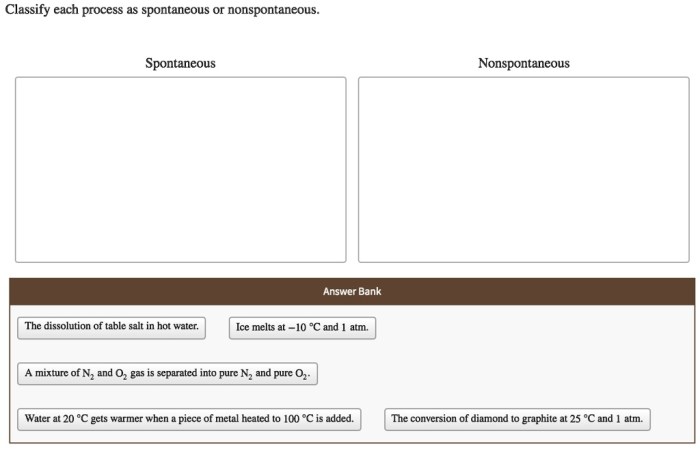
Spontaneity refers to the tendency of a chemical reaction to proceed without external intervention. Spontaneous processes occur with a decrease in free energy, releasing energy in the form of heat or work.
Examples of spontaneous processes include:
- Dissolution of sugar in water
- Rusting of iron
- Combustion of fuels
Nonspontaneous processes, on the other hand, require an external input of energy to occur. Examples of nonspontaneous processes include:
- Formation of water from hydrogen and oxygen
- Freezing of water
- Electrolysis of water
Factors that determine the spontaneity of a reaction include:
- Change in free energy
- Entropy
- Enthalpy
- Temperature
Thermodynamics and Spontaneity: Classify Each Process As Spontaneous Or Nonspontaneous.
Thermodynamics plays a crucial role in understanding spontaneity. Entropy, a measure of disorder, and enthalpy, a measure of heat flow, influence the spontaneity of a reaction.
The Gibbs free energy equation, ΔG = ΔH – TΔS, relates spontaneity to these thermodynamic properties:
- ΔG< 0: Spontaneous reaction
- ΔG > 0: Nonspontaneous reaction
- ΔG = 0: Equilibrium
Examples of how thermodynamics can be used to predict spontaneity include:
- Calculating the free energy change of a reaction using enthalpy and entropy data
- Determining the spontaneity of a reaction at different temperatures
- Predicting the direction of a chemical equilibrium
Reaction Kinetics and Spontaneity
Reaction kinetics, the study of reaction rates, is also related to spontaneity. The activation energy, the minimum energy required for a reaction to occur, plays a key role in spontaneity.
Fast reactions with low activation energies are more likely to be spontaneous than slow reactions with high activation energies. Examples of how reaction kinetics can be used to understand spontaneity include:
- Measuring the activation energy of a reaction to determine its spontaneity
- Predicting the rate of a reaction and its spontaneity under different conditions
- Designing catalysts to increase the spontaneity of reactions
Applications of Spontaneity
Understanding spontaneity has practical applications in various fields, including:
Chemical Engineering:
- Designing chemical processes that maximize spontaneity
- Predicting the feasibility of chemical reactions
Environmental Science:
- Understanding the spontaneity of environmental processes
- Predicting the impact of pollutants on ecosystems
Biological Processes:
- Analyzing the spontaneity of metabolic reactions
- Predicting the direction of biochemical pathways
Common Queries
What is the driving force behind spontaneous reactions?
Spontaneous reactions are driven by a decrease in the system’s Gibbs free energy, which reflects a favorable balance between enthalpy and entropy changes.
How does temperature affect spontaneity?
Temperature can influence spontaneity by altering the entropy and enthalpy changes of a reaction. Higher temperatures generally favor reactions with positive entropy changes.
What is the role of activation energy in spontaneity?
Activation energy represents the energy barrier that must be overcome for a reaction to occur. Reactions with lower activation energies are more likely to be spontaneous.